Our report on the discovery of a new suite of extracellular matrix (ECM) molecules was published in the latest issue of Molecular Biology of the Cell. The report shows how a set of unconventional extracellular proteins, called the MLTN family, play a critical role in development of the model nematode C. elegans. Related ECM fibrils may contribute to the strength and flexibility of the exoskeletons of virtually all nematodes. In this context, our ongoing studies of the MLTN family may expedite the development of more effective drugs urgently needed to combat diseases caused by parasitic nematodes that currently affect approximately 2.9 billion people living primarily in tropical regions.
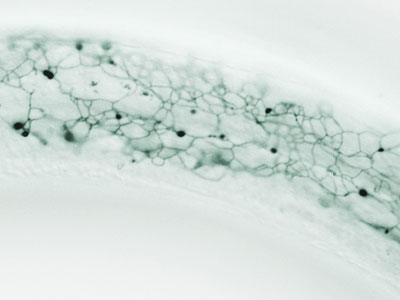
Nematodes, arthropods, and other ecdysozoans grow by periodically shedding and rebuilding their external skeletons (cuticles). Although ecdysozoans dominate the animal kingdom, the mechanisms by which cuticles are removed and remade are not well understood in any organism. The mlt-10 gene, which encodes the defining member of the large MLTN family, was isolated in a genetic screen for mutants unable to molt. Hallmarks of the MLTN proteins include the “Molting Cycle” Domain and distinctive proline-rich repeats. The annotated genomes of all nematode species encode multiple family members. In addition, similar tandem repeats were found in uncharacterized proteins of frogs, mice, and humans, suggesting that the cassette performs an evolutionarily ancient function such as macromolecular assembly. Consistent with this view, MLT‑10::mCherry fusion proteins were made in the epidermis and incorporated into newly-made cuticles each time worms molted. The fusion proteins were detected on the surface of transgenic worms, as shown in the image above.
Further studies revealed that mutations in mlt-10 were associated with abnormalities in three distinct but interconnected developmental processes: shedding larval cuticles, patterning adult cuticles, and forming the underlying epidermis. Based on these findings, we proposed that MLTN proteins co-assemble into a variety of ECM macromolecules that together serve as both structural and instructive components of nematode cuticles. MLTN macromolecules likely co-exist, and perhaps interact, with collagens and other ECM proteins that are also used to make human skin and connective tissue.
Full article:
Meli, V.S., Osuna, B., Ruvkun, G. and Frand, A.R. (2010) MLT-10 defines a family of DUF644 and proline-rich repeat proteins involved in the molting cycle of Caenorhabditis elegans. Molecular Biology of the Cell 15:1648-61.
PubMed | Article| PDF